Macrophages are innate immune cells with well-established roles in tissue homeostasis, primary response to pathogens, coordination of adaptive immunity, and even wound repair. Macrophages accomplish these varied roles by adapting their gene and protein expression programs in response to endogenous and exogenous environmental cues, such as cytokines and pathogen-associated molecular patterns.
Principal Investigator
Jörg Hamann, PhD
The ability of macrophages to change their gene and protein signatures falls under the umbrella of a developing concept: macrophage plasticity. Plasticity is naturally at the basis of macrophage heterogeneity in basal and inflammatory conditions but also at the basis of worldwide efforts to treat diseases by subverting aberrant macrophage activation. Macrophage-mediated inflammation is increasingly recognized to contribute to chronic inflammatory disorders, such as chronic obstructive pulmonary disease, severe asthma, rheumatoid arthritis, and multiple sclerosis. We are studying the cellular program and function of human tissue macrophages in health in disease. To this we collaborate with Dr. Kris Reedquist, Dr. Marco van Eijk, Dr. Rene Jonkers (all AMC, Amsterdam), Dr. Inge Huitinga (NIN, Amsterdam), and Dr. Fernando Martinez (University of Oxford, UK).
Adhesion-GPCRs
G protein-coupled receptors (GPCRs) represent the largest superfamily of receptors in the human genome. Present on every cell and responding to a plethora of stimuli, GPCRs are involved in a great variety of physiological processes. Phylogenetic comparison has led to the GRAFS classification, which divides GPCRs into five classes called Glutamate, Rhodopsin, Adhesion, Frizzled/taste, and Secretin. The adhesion class comprises 33 members in humans with a broad distribution in embryonic and larval cells, cells of the reproductive tract, neurons, leukocytes, and various tumor cells.
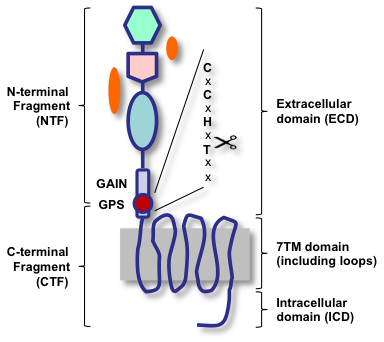
Adhesion-GPCRs possess a juxtamembranous GPCR proteolysis site (GPS) that facilitates autocatalytic processing into an extracellular N-terminal fragment (NTF) and a 7TM/cytoplasmic C-terminal fragment (CTF), which subsequently remain associated. Only recently, it became clear that the autoproteolysis site is part of a much larger ~320-residue GAIN (GPCR autoproteolysis-inducing) domain that forms a non-covalently associated heterodimer upon proteolysis. The cartoon shows the general design and terminology of Adhesion-GPCRs based on cleavage (left) and topology (right).
Despite their wide distribution and some dramatic phenotypes resulting from lack or gain of function, Adhesion-GPCRs are ‘functional orphans’. Little is known regarding how these unusual GPCRs are activated, which is due to uncertainty about the role of the identified binding partners and the cooperation between the NTF and the CTF of the receptors. Recent studies imply that adhesion through the NTF and signaling through the CTF may be separated activities and demonstrate the ability of Adhesion-GPCRs to modulate the activity of other receptors in a ligation-independent manner.
After having cloned and deorphanized CD97 in the mid 90th, work on Adhesion-GPCRs expressed by cells of the immune system became a major research focus at our laboratory. We have studied the structure, expression, evolution, ligand specificity, and function of these receptors in humans and mice, thereby using molecular tools and mouse models developed in our laboratory. Using the CD97-CD55 interaction as a model, we recently proved the interaction of an Adhesion-GPCR with its endogenous binding partner in vivo. Our findings strengthen the hypothesis that Adhesion-GPCRs adhere and signal independently through their two subunits.
Our current work focuses on the function and mechanism of action of Adhesion-GPCRs on immune cells, thereby exploring CD97 and GPR56 as models. We have close and long-lasting collaborations with Prof. Siamon Gordon (University of Oxford, UK), Prof. Gabriela Aust (University of Leipzig, Germany), Prof. Hsi-Hsien Lin (Chang Gung University, Taiwan), Dr. Martin Stacey (University of Leeds, UK), Dr. Tobias Langenhan (University of Würzburg, Germany), and other members of the international Adhesion-GPCR Consortium (AGC, www.adhesiongpcr.org). Our final goal is to utilize the potential of Adhesion-GPCRs for human health.
Group members
Jörg Hamann, PhD
Kirstin Heutinck, PhD
Cheng-Chih Hsiao, MSc
Hanneke de Kort, PhD
Former group members:
Martijn van de Garde, MSc
Dennis Flierman, PhD
Robert Hoek, PhD
Else Kop, MD, PhD
Olga Karpus, PhD
Mark Kwakkenbos, PhD
Mourad Matmati, PhD
Henrike Veninga, PhD
Walter Pouwels, Ing
Current undergraduate students:
-
Students are welcome to perform research internships within our group. We permanently have projects available for medical students and students in the master programs Immunology, Medical Biochemistry, Medical Biology and Biology.
Selected publications
Hamann, J., G. Aust, D. Araç, F.B. Engel, C. Formstone, R. Fredriksson, R.A. Hall, B.L. Harty, C. Kirchhoff, B. Knapp, A. Krishnan, I. Liebscher, H.H. Lin, D.C. Martinelli, K.R. Monk, M.C. Peeters, X. Piao, S. Prömel, T. Schöneberg, T.W. Schwartz, K. Singer, M. Stacey, Y.A. Ushkaryov, M. Vallon, U. Wolfrum, M.W. Wright, L. Xu, T. Langenhan, H.B. Schiöth (2015). International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharm. Rev.67: 338-67.
Van de Garde, M.D.B., F.O. Martinez, B.N. Melgert, M.N. Hylkema, R.E. Jonkers and J. Hamann (2014). Chronic exposure to glucocorticoids shapes gene expression and modulates innate and adaptive activation pathways in macrophages with distinct changes in leukocyte attraction. J. Immunol. 192: 1196-208. Highlighted in Nature Rev. Immunol. 14: 66 (2014).
Melief, J., K.G. Schuurman, M.D. van de Garde, J. Smolders, M. van Eijk, J. Hamann and I. Huitinga (2013). Microglia in normal appearing white matter of multiple sclerosis are alerted but immunosuppressed. Glia 61: 1848-61.
Smolders, J., E.B.M. Remmerswaal, K.G. Schuurman, J. Melief, C.G. van Eden, R.A.W. van Lier, I. Huitinga and J. Hamann (2013). Characteristics of differentiated CD8+ and CD4+ T cells present in the human brain. Acta Neuropathol. 126: 525-35.
Aust, G., C. Kerner, S. Gonsior, D. Sittig, H. Schneider, P. Buske, M. Scholz, N. Dietrich, S. Oldenburg, O.N. Karpus, J. Galle, S. Amasheh and J. Hamann (2013). Mice overexpressing CD97 in intestinal epithelial cells provide a unique model for mammalian postnatal intestinal cylindrical growth. Mol. Biol. Cell 24: 2256-68.
Langenhan, T., G. Aust and J. Hamann (2013) Sticky signaling – Adhesion class G protein-coupled receptors take the stage. Sci. Signal. 6: re3.
Karpus, O.N., H. Veninga, R.M. Hoek, D. Flierman, J.D. van Buul, C.C. vandenAkker, E. vanBavel, M.E. Medof, R.A.W. van Lier, K.A. Reedquist and J. Hamann (2013). Shear stress-dependent downregulation of the adhesion-G protein-coupled receptor CD97 on circulating leukocytes upon contact with its ligand CD55. J. Immunol. 190: 3740-8.
Heutinck, K.M., A.T. Rowshani, J. Kassies, N. Claessen, F.J. Bemelman, E. Eldering, R.A.W. van Lier, S. Florquin, I.J.M. ten Berge and J. Hamann (2012). The viral dsRNA sensors TLR3, MDA5 and RIG-I induce pro-inflammatory, anti-viral and pro-apoptotic responses in human renal tubular epithelial cells. Kidney Int. 82: 664-75.
Melief, J., N. Koning, K.G. Schuurman, M.D. van de Garde, J. Smolders, R.M. Hoek, M. van Eijk, J. Hamann and I. Huitinga (2012). Phenotyping primary human microglia: Tight regulation of LPS responsiveness. Glia 60: 1506-17.
Gordon, S., J. Hamann, H.H. Lin and M. Stacey (2011). Celebrating 30 years: F4/80 and the related adhesion-GPCRs. Eur. J. Immunol. 41: 2470–525.
Hoek, R.M., D. de Launay, E.N. Kop, A.S. Yilmaz-Ellis, F. Lin, K.A. Reedquist, J.S. Verbeek, M.E. Medof, P.P. Tak and J. Hamann (2010). Deletion of either CD55 or CD97 ameliorates arthritis in mouse models. Arthritis Rheum. 62: 1036-42.
Veninga, H., S. Becker, R.M. Hoek, M. Wobus, E. Wandel, J. van der Kaa, M. van der Valk, A.F. de Vos, H. Haase, B. Owens, T. van der Poll, R.A.W. van Lier, J.S. Verbeek, G. Aust and J. Hamann (2008). Analysis of CD97 expression and manipulation: antibody treatment but not gene targeting curtails granulocyte migration. J. Immunol. 181: 6574-83.
Hamann, J., N. Koning, W. Pouwels, L. Ulfman, M. van Eijk, M. Stacey, H.H. Lin, S. Gordon and M.J. Kwakkenbos (2007). EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur. J. Immunol. 37: 2797-2802.
Kwakkenbos, M.J., M. Matmati, O. Madsen, W. Pouwels, Y. Wang, R. Bontrop, P.J. Heidt, R.M. Hoek and J. Hamann (2006). An unusual mode of concerted evolution of the EGF-TM7 receptor chimera EMR2. FASEB J. 20: 2582-4.
Kop, E.N., M.J. Kwakkenbos, G.J.D.Teske, M.C. Kraan, T.J. Smeets, M. Stacey, H.H. Lin, P.P. Tak and J. Hamann (2005). Identification of the epidermal growth factor-TM7 receptor EMR2 and its ligand dermatan sulfate in rheumatoid synovial tissue. Arthritis Rheum. 52: 442-50.
Leemans, J.C., A.A. te Velde, S. Florquin, R.J. Bennink, K. de Bruin, R.A.W. van Lier, T. van der Poll and J. Hamann (2004). The EGF-TM7 receptor CD97 is required for neutrophil migration and host defense. J. Immunol. 172: 1125-31.
Hamann, J., M.J. Kwakkenbos, E.C. de Jong, H. Heus, A.S. Olsen and R.A.W. van Lier (2003). Inactivation of the EGF-TM7 receptor EMR4 after the Pan-Homo divergence. Eur. J. Immunol. 33: 1365-71.
Hamann, J., J.O. Wishaupt, R.A.W. van Lier, T.J. Smeets, F. Breedveld and P.P. Tak (1999). Expression of the activation antigen CD97 and its ligand CD55 in rheumatoid synovial tissue. Arthritis Rheum. 42: 650-8.
Hamann, J., C. Stortelers, E. Kiss‑Toth, B. Vogel, W. Eichler and R.A.W. van Lier (1998). Characterization of the CD55 (DAF)‑binding site on the seven‑span transmembrane receptor CD97. Eur. J. Immunol. 28: 1701-7.
Hamann, J., B. Vogel, G.M.W. van Schijndel and R.A.W. van Lier (1996). The seven‑span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J. Exp. Med. 184: 1185-9.
Hamann, J., W. Eichler, D. Hamann, H.M.J. Kerstens, P.J. Poddighe, J.M.N. Hoovers, E. Hartmann, M. Strauss and R.A.W. van Lier (1995). Expression cloning and chromosomal mapping of the leukocyte activation antigen CD97, a new seven‑span transmembrane molecule of the secretin receptor superfamily with an unusual extracellular domain. J. Immunol. 155: 1942-50.
Hamann, J., H. Fiebig and M. Strauss (1993). Expression cloning of the early activation antigen CD69, a type II integral membrane protein with a C‑type lectin domain. J. Immunol. 150: 4920-7.
For a full list with publication klick here.
Contact
For further information about our research and opportunities for work or collaborations, you can contact Dr. Jörg Hamann
+31 (0)20 566 6080