In 2018 the Core Facility Metabolomics obtained a NWO middelgroot grant for the acquisition of a Bruker Trapped Ion Mobility Spectrometry (TIMS) – Time Of Flight (TOF) or TimsTOF in short. This mass spectrometer introduces next generation high-resolution ion mobility separation combined with an well-established high-resolution QTOF mass spectrometer. As regular chromatographic separation is not able to separate all classes of molecules an additional separation technique is required, especially for isomers. Ion mobility offers such extension to mass spectrometry and delivers information about the three-dimensional structure of an ion. Ion mobility is capable of separating isomers. In Trapped Ion Mobility Spectrometry (Figure 1A-B), ions are propelled through the TIMS tunnel by a gas flow. An electrical field controls each ion from moving beyond a position defined by the ion’s mobility (depending on its 3D structure), where the push it experiences from the gas flow matches the force of the electrical field. Ramping down the electrical field allows selectively releasing ions from the TIMS tunnel according to their mobility.
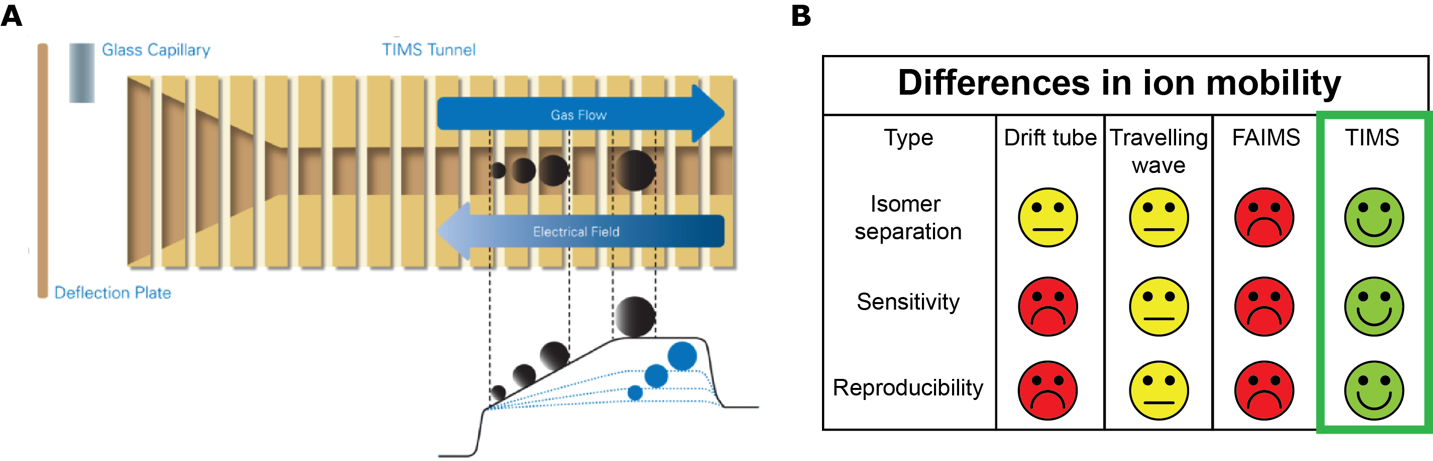
Figure 1. Basis and characteristics of Trapped Ion Mobility Spectroscopy. (A) Trapped Ion Mobility Spectrometry (TIMS): each ion is trapped in the TIMS tunnel at the point where the force of a gas flow matches the opposing force of an increasing electrical field in the tunnel. Ions are portrayed aligned along the electrical field gradient, for selective release the electrical field is ramped down. (B) TIMS ion mobility compared to currently used ion mobility techniques. TIMS is superior on all fronts.
The resolution of the TIMS can be adjusted depending on the goal of the experiment. Using a lower TIMS resolution, metabolomics/fluxomics samples can first be broadly surveyed, after which a higher TIMS resolution can be used for detailed examination of selected compounds. In addition to the high resolution, TIMS has improved sensitivity which means that less sample is “lost” during the measurement and hence detection is improved. Such characteristics make timsTOF unique in the current ion mobility landscape (Figure 1B). This new type of ion mobility is ideally suited for metabolomics as it will increase the number of identifiable metabolites, enhance compound identification confidence, and in some cases, obviates the need for chromatographic separation, which further increases efficiency and analysis throughput. This is particularly evident for isotope distribution analysis, which is crucial for the labelled isotope metabolomics studies (fluxomics) as mentioned above in case 2. Importantly, the data structure of this machine is an open format which allows us to integrate the data output with existing home-made pre-processing scripts, which is a considerable advantage allowing fast integration with our existing bioinformatic pipelines.
Some examples of ion mobility performed on the timsTOF are described below:
In the first example, the carbohydrates galactose-1-phosphate and glucose-1-phosphate are isomers (Figure 2) which are difficult to separate using regular chromatography. These isomers can be separated using ion mobility, but current ion mobility techniques cannot solve this issue satisfactory as they both lack sensitivity and resolving power. In our preliminary experiments, we performed timsTOF measurements on these carbohydrates using the high-resolution ion mobility mode (imeX Ultra mode). We show that galactose-1-phosphate and glucose-1-phosphate can be separated and individually identified/quantified (Figure 3).
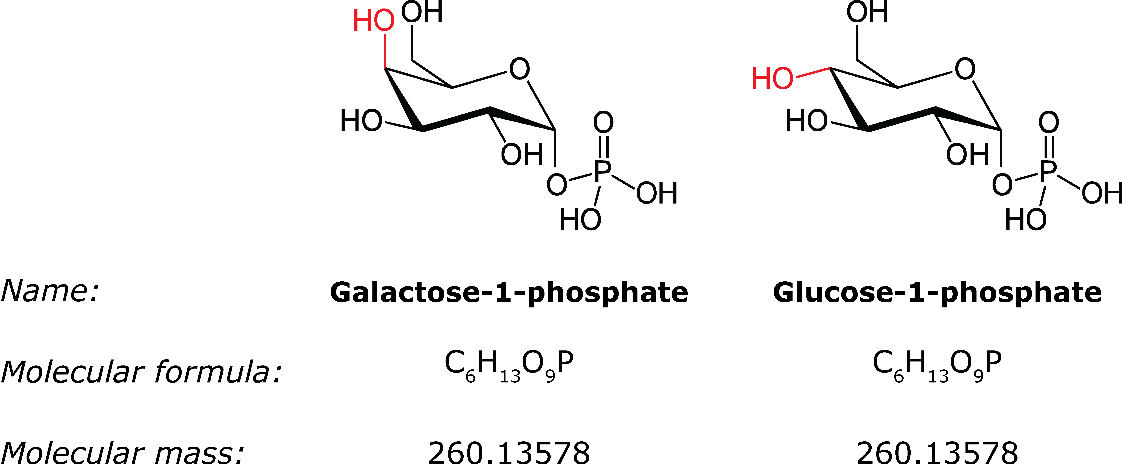
Figure 2. Structure and information of galactose-1-phosphate and glucose-1-phosphate. These metabolites have a different structure but the same molecular formula and exact mass, they are isomeric compounds.
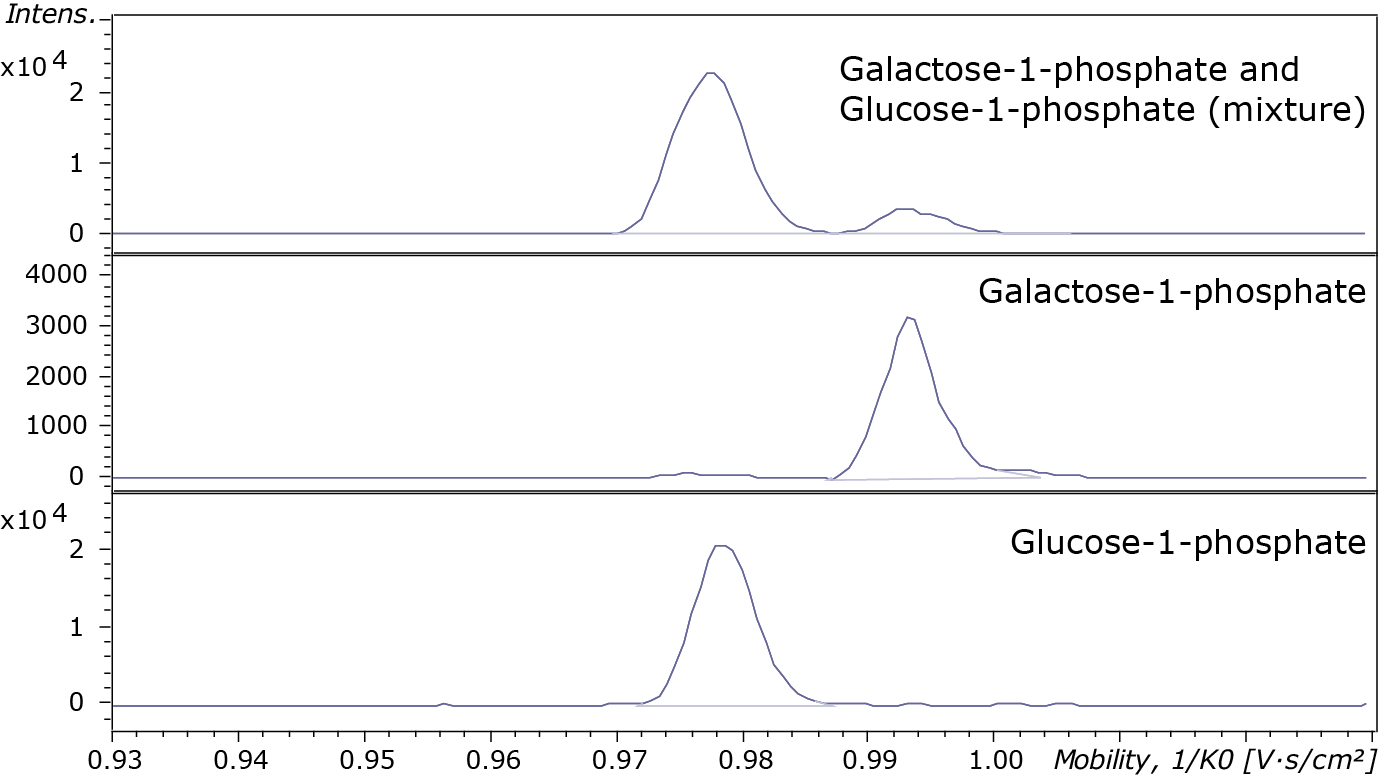
Figure 3. Separation of isomeric metabolites using timsTOF. Carbohydrates with the same elemental composition (C6H13O9P) and mass (m/z 259.0224) galactose-1-phosphate and glucose-1-phosphate, but differ in their three-dimensional structure. In imeX Ultra mode these two carbohydrates could be separated and individually identified/quantified with ion mobility resolution up to 182.
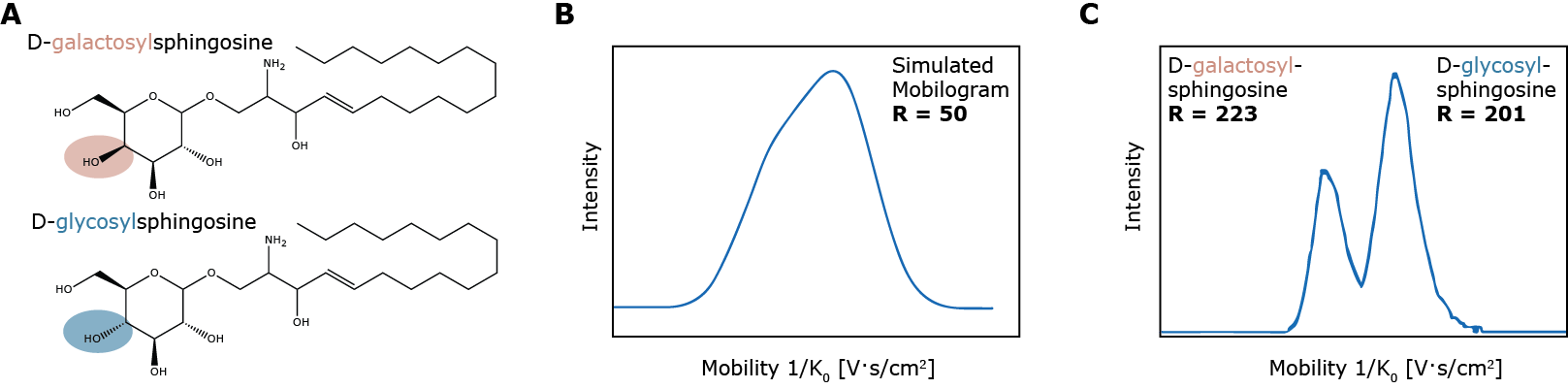
In the second example, timsTOF is used to separate isomeric glycosphingolipid species D-galactosyl- and D-glycoylsphingosine (Figure 4A). These very similar molecules, differing only in the orientation of the hydroxylgroup on the sugar headgroup, are not sufficiently separated on current ion mobility systems (Figure 4B) which have an average resolution of 50 whereas the high resolution of TIMS (>200) illustrates the utility of this novel technique in lipidomic analysis of complex lipids (Figure 4C).